

Antimicrobial test results


Anti-microbial test
List of antimicrobial test
Anti-microbial effect on plastic molding
Test method: JIS Z 2801 / ISO 22196
Bacteria used: Escherichia coli.
| Sample | Number of cells | Reduction rate(%) | ||
|---|---|---|---|---|
| Beginning | 24 hours later | |||
| PP plate with 0.1% IONPURE | 1×105 | <1×102 | >99.999% | |
| Blank | 1×105 | 5×107 | - | |
Bacteria used: Staphylococcus aureus
| Sample | Number of cells | Reduction rate(%) | ||
|---|---|---|---|---|
| Beginning | 24 hours later | |||
| PP plate with 0.1% IONPURE | 1×105 | <1×102 | >99.99% | |
| Blank | 1×105 | 6×106 | - | |
Antimicrobial efficacy with fiber
Test method : JIS L 1902 / ISO 20743
Bacteria used : Staphylococcus aureus
| Sample | Number of cells | Reduction rate(%) | ||
|---|---|---|---|---|
| Beginning | 18 hours later | |||
| PP fiber with IONPURE 0.3% | 8×105 | 1×102 | 99.99% | |
| Blank | 8×105 | 4×106 | - | |
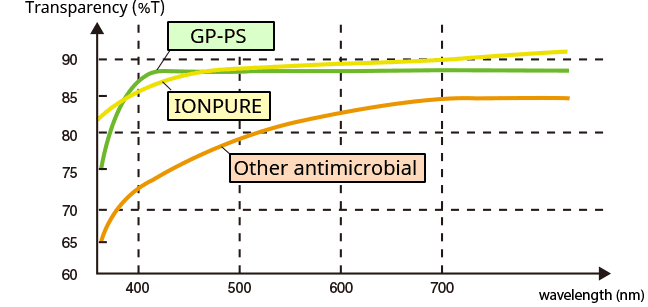
Transparency
Transmitting curve of GPPS with IONPURE
Test method : JIS Z 2801 / ISO 22196
Bacteria used: Escherichia coli
| Sample | Number of cells | Reduction rate(%) | ||
|---|---|---|---|---|
| Beginning | 24 hours later | |||
| GP-PS plate with 0.15% IONPURE | 2×105 | <1×102 | >99.999% | |
| Blank | 2×105 | 1×107 | - | |
Durability
Washing deterioration test
Test method : JIS L 1902 / ISO 20743
PP fiber with IONPURE
| Sample | Number of cells | Reduction rate(%) | ||
|---|---|---|---|---|
| Beginning | 18 hours later | |||
| PP fiber with IONPURE (non washed) | 3×104 | <2×102 | >99.99% | |
| PP fiber with IONPURE (50 wash-cycles) | 3×104 | <2×102 | >99.99% | |
| Blank | 3×104 | 1×107 | - | |
PET fiber with IONPURE
| Sample | Number of cells | Reduction rate(%) | ||
|---|---|---|---|---|
| Beginning | 18 hours later | |||
| PP fiber with IONPURE (non washed) | 3×104 | <2×102 | >99.99% | |
| PP fiber with IONPURE (50 wash-cycles) | 3×104 | 4×102 | 99.99% | |
| Blank | 3×104 | 2×107 | - | |
The properties of plastics
Strength test for PC with IONPURE
| Test item | Test method | Material | ||
|---|---|---|---|---|
| Blank PC plate | PC plate with 0.5% IONPURE | |||
| Tensile strength 50mm/min | ASTM D-638 (kg/cm2) |
At yield point | 642 | 639 |
| At break point | 746 | 662 | ||
| Bending strength 3mm/min | ASTM D-790(kg/cm2) | 924 | 931 | |
| Impact strength | ASTM D-256(kg・cm/cm2) | 82.60 | 83.86 | |
| Heat transforming temperature load 18.5kg | ASTM D-648 (℃) | 132.4 | 132.8 | |
Safety
List of safety data
| Acute oral toxicity | There was no case of abnormal reaction or death by single oral administration of 2,000mg/kg of IONPURE to mice. |
|---|---|
| Primary skin irritation | No skin irritation response was observed in albino rabbits during or after the skin irritation test suggested in the OECD Chemical Substance Toxicity Test Guidance.(1981) |
| Mutagenicity | The mutagenicity test based on the bulletin No.77 of the Labor Ministry of Japan (1st Sep.1989) resulted in no increase in reverse mutation and mutagenicity was confirmed to be negative. |
| Skin sensitization | The skin sensitization test using maximization method demonstrated no skin sensitization on guinea pigs. |
"IONPURE" is a trademark of Ishizuka Glass Co., Ltd.
Contact
- Please feel free to contact us
if you have any inquiries regarding
our products and business. - Contact



