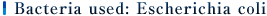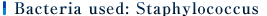For Cosmetic
Possible to maintain the quality of cosmetic without using paraben.

For Water Based Coating
Possible to achieve antimicrobial efficacy at low dosage level.

For ABS resin
Possible to achieve antimicrobial efficacy at low dosage level.
Following table shows industrial applications example of IONPURE.
| Field | Application |
|---|---|
| Daily necessities | Chopping board, Disposable gloves, Bowl, Kitchenware, Kitchen knife handle, Duster, Washbowl, Toothbrush, Wrapping film, Canteen, Emergency water tank, Hairbrush, Toilet seat, Cleaner etc. |
| Electric application | Refrigerator, Dish washer, Dish dryer, Mobile phone, Water cleaner, Iron, Gas table, Remote controller etc. |
| Automobile parts | Steering wheel, Shift knob, Switches, Assist grip, Parking break lever etc. |
| Fiber | Socks, Pantyhose, Tights, Insole, Bathing suit, Toilet cobber, Towel, Carpet, Pillow, Sheets etc. |
| Building materials | Wall paper, flooring materials (P tile, Floorings), Mat, Artificial marble (For kitchen counter, Washstand and Bathtub) etc. |
| Medical | Measuring instrument, Hospital floor, Curtain, Stationery, White dress, Gauze mask etc. |
| Food relatated | Conveyor belt, Floor tray etc. |
| Others | Contact lens utility (Lens keeper, Lens holder), Cosmetics, Credit card etc, |
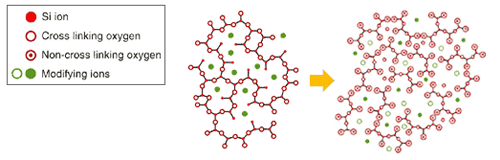
Glass is generally considered as a material with high chemical inertness due to its strong network structure. However, it is possible to lower this chemical inertness by continuously altering its structure especially in water.
On the other hand, glass has another interesting property, which retains metal as ions. Combination of this property and the above technology has enabled to create glass with low chemical inertness, which can also retain anti-microbial metal ions such as silver and copper. With the presence of water or moisture, the glass will release these metal ions gradually to function as anti-microbial material.
it is well known that these ion have antimicrobial effect. For example, silverware and copperware has been known to prevent decay of water and food since the ancient times.
- Freely design the anti-microbial effect and life span according to using purpose.
- Adaptable to all kind of resin in various grades.
- High performance at low concentration.
- Keep up transparency of resin such as PP, PC, ABS, AS.
- Adapt every molding and spinning as its heat-resistance is more than 500℃.
- Keep up high performance after washing and warm water environment.
- High level of safety.
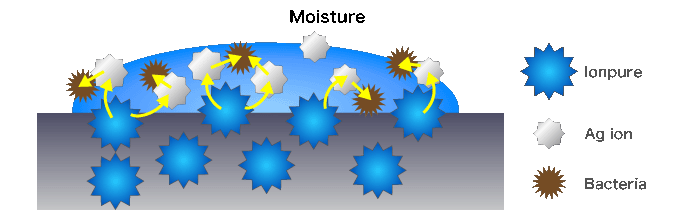
With the presence of moisture, IONPURE releases a few Ag ions gradually. Ag ion has an ability to strongly bind to cellular enzyme of microbes and inhibit enzyme activity of cell wall, membrane, and nucleic acids. In a word, as microbes charged with minus on their surface, Ag ions that have plus are drawn toward microbes, and disturb their electric balance. so that microbes are burst their cell wall and extinguished.
Otherwise as Ag ions are taken into microbes, they react and bond to the cellular enzyme microbes. this inhibits enzyme activity and multiplication of microbes, thus extinguishing the microbes.
IONPURE can apply to the uses for compounding or coating or dipping of resin and fiber.
| Sample | Number of cells | Log | Log (Blank) -Log (Samples) |
|
|---|---|---|---|---|
| Beginning | 24 hours later | |||
| PP plate with 0.1% IONPURE |
1×105 | <1×102 | <2.0 | >5.7 |
| Blank | 1×105 | 5×107 | 7.7 | - |
Note 1)Test method: in accordance with JIS Z 2801
| Sample | Number of cells | Log | Log (Blank) -Log (Samples) |
|
|---|---|---|---|---|
| Beginning | 24 hours later | |||
| PP plate with 0.1% IONPURE |
1×105 | <1×102 | <2.0 | >4.8 |
| Blank | 1×105 | 6×107 | 6.8 | - |
| Sample | Number of cells | Log | Log (Blank)-Log (Samples) | |
|---|---|---|---|---|
| Beginning | 18 hours later | |||
| PP fiber with 0.3% IONPURE | 8×105 | 1×102 | 2.0 | 4.6 |
| Blank | 8×105 | 4×106 | 6.6 | - |
| Sample | Number of cells | Log | Log (Blank)-Log (Samples) | |
|---|---|---|---|---|
| Beginning | 18 hours later | |||
| PET fiber with 0.5% IONPURE | 3×104 | <1×102 | <2.0 | >5.0 |
| Blank | 3×104 | 1×107 | 7.0 | - |
Note 1) Bacteria used: Staphylococcus IFO 12732
Note 2)Test method: in accordance with JIS L 1902
Fiber containing Ionpure can keep up anti-microbial effect even after washing.
| Sample PP fiber | Number of cells | Log | Log (Black) -Log (Samples) |
|
|---|---|---|---|---|
| Beginning | 18 hours later | |||
| IONPURE non washed | 3×104 | <2×102 | <2.3 | >4.7 |
| IONPURE 50 wash-cycles | 3×104 | <2×102 | <2.3 | >4.7 |
| Blank | 3×104 | 1×107 | 7.0 | - |
Note 1) Bacteria used: Staphylococcus aueus IFO 12732
Note 2) Test method: in accordance with JIS L 1902
| Sample PP fiber | Number of cells | Log | Log (Black) -Log (Samples) |
|
|---|---|---|---|---|
| Beginning | 18 hours later | |||
| IONPURE non washed | 3×104 | <2×102 | 2.3 | >5 |
| IONPURE 50 wash-cycles | 3×104 | <4×102 | 2.6 | 4.7 |
| Blank | 3×104 | 2×107 | 7.3 | - |
Heat-resistance of IONPURE is more than 500℃. as it is inorganic. It is possible to apply to every molding and spinning. Moreover, it can apply heat-resistant container made from resin.
IONPURE is not discolored by compounding and with age, as it is can control the speed of releasing metal ions which have anti-microbial effect.
To be enable to design freely it's life span of effect by combining glass composition and metal ions having anti-microbial effect is only IONPURE.
| Sample PP fiber | Number of cells | Log | Log (Black)-Log (Samples) | |
|---|---|---|---|---|
| Beginning | 24 hours later | |||
| Boiled 500 hours | 1×105 | 1×104 | 4 | 3 |
| Boiled 1000 hours | 1×105 | 3×104 | 4.4 | 2.6 |
| Blank | 1×105 | 1×107 | 7.0 | - |
Note 1) Used bacteria: Escherichia coli NBRC 3972
Note 2) Test method: in accordance with JIS Z 2801
In general, adding inorganic material to transparent resin such as PP, PVC, ABS, PS, cause the turbidity. but Ionpure, as it is glass, can keep up the transparency of resin to control its refractive index.
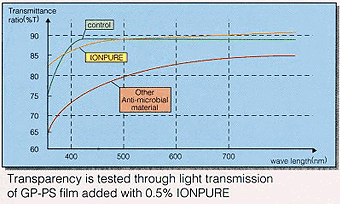
| Sample | Number of cells | Log | Log (Blank)-Log (Samples) | |
|---|---|---|---|---|
| Beginning | 24 hours later | |||
| GP-PS with 0.15% IONPURE | 2×105 | <1×102 | <2.0 | >5.0 |
| Blank | 2×105 | 1×107 | 7.0 | - |
Note 1)Bacteria used: Escherichia coli NBRC 3972
Note 2)Test method: in accordance with JIS Z 2801
IONPURE does not effect the properties of plastics.
| Strength test | Test method | Material | ||
|---|---|---|---|---|
| Blank PC plate | PC plate with 0.5% IONPURE |
|||
| Tensile strength 50mm/min | ASTM D-638 (kg/cm2) |
At yield point | 642 | 639 |
| At break point | 746 | 662 | ||
| Bending strength 3mm/min | ASTM D-790(kg/cm2) | 924 | 931 | |
| Impact strength | ASTM D-256(kg・cm/cm2) | 82.60 | 83.86 | |
| Heat transforming temperature load 18.5kg |
ASTM D-648 (℃) | 132.4 | 132.8 | |
IONPURE has acquired various safety data for each grade of the product. Following are examples of safety data.
- Acute oral toxicity
There was no case of abnormal reaction or death by single oral administration of 5000mg/kg(2000mg/kg) of IONPURE to mice. - Primary skin irritation
No skin irritation response was observed an albino rabbits during or after the skin irritation test suggested in the OECD Chemical Substance Toxicity Test Guidance.(1981) - Mutagenicity
The mutagenicity test based on the bulletin No.77 of the Labor Ministry of Japan (1st Sep.1989)resulted in no increase in reverse mutation and mutagenicity was confirmed to be negative. - Skin sensitization
The skin sensitization test using maximization method demonstrated no skin sensitization on guinea pigs. - Inhibition of colony forming
In accordance with "Guidelines for Biological Tests of Medical Devices" (No.99), the value was 0.51mg/ml for inhibition of 50% colony forming (IC50).
In addition to above test results, eye irritancy test, photo toxicity test, and repeat insult patch test have been performed for other grades of IONPURE. Also, other products containing IONPURE have exhibited excellent safety data in human patch test and quality assurance test for food containers and appliances based on Food Hygiene Law of Japan.
'Ion pure' is approved in different kinds of legislation in worldwide.
-
1.EPA Approved
Ion pure is FIFRA (The Federal Insecticide, Fungicide, and Rodenticide Act) approved.
EPA approved Ion pure can be used for applications related to non-food contact, food contact, and drinking water applications. For example, polymer incorporated applications, tupperware, artificial counter top, drinking water filter, and ice machine applications etc. It is prohibited to distribute and market an antimicrobial agent without EPA approvals in the USA.Food Contact & Drinking Water - ION PURE WPA (73148-1)
- ION PURE IPL (73148-3)
Non-Food Contact - ION PURE ZAF (73148-2)
- ION PURE IZA (73148-5)
- ION PURE ZAF HS (73148-6)
- ION PURE ZAF MS (73148-7)
- ION PURE IPI (73148-8)
-
2.FDA Approved
Ion pure is FDA (Food and Drug Administration) approved.
Ion pure can be applied to any polymers used for industrial or food packaging use application.
Listed applications are, food wrapping applications, tray, bottles, containers used for delivery of food- FCN No. 000432 ION PURE IPL
- FCN No. 000433 ION PURE WPA
- FCN No. 000476 ION PURE IZA
-
3.NSF
Ion pure is listed under NSF (National Sanitation Foundation) approved substance under NSF Standard 51.
With this registration, Ion pure can be applied in polymeric applications which are used in food contact settings.
Possible applications are, dish washer, ice machine, refrigerator, kitchen utensils, belt conveyorNSF Standard 51
- ION PURE WPA
- ION PURE IPL
- ION PURE IZA
-
1.BPR (Biocidal Product Regulation) Regulation (EU) 528/2012
BPR became effective after 1 September 2013. All biocidal products require an authorization before they can be placed on the market. In addition, the active substances contained in the biocidal product must be approved previously. Dossiers of Ion pure have already been submitted in 2007 and 2008, and Ishizuka Glass is directly involved in this evaluation process.
→BPR evauation is still on going. Additional information will be updated as evaluation goes on.
Article 95 List: Silver Phosphate Glass
Active substances which are not listed under Article 95 listing after 1 September 2015 can no longer be marketed in EU.Ishizuka is listed under Article 95 listActive Substance: Silver Phosphate Glass Listed as: Substance & Product Supplier -
2. EFSA (European Food Safety Authority)
Ion pure has received an EFSA opinion concluding this substance can be applied in food contact applications.
Ion pure grades received an EFSA opinion
- ION PURE IPL (86432)
- ION PURE WPA (86432-20)
- ION PURE ZAF (86432-40)